The design principle of the biological space expansion pattern diagram.
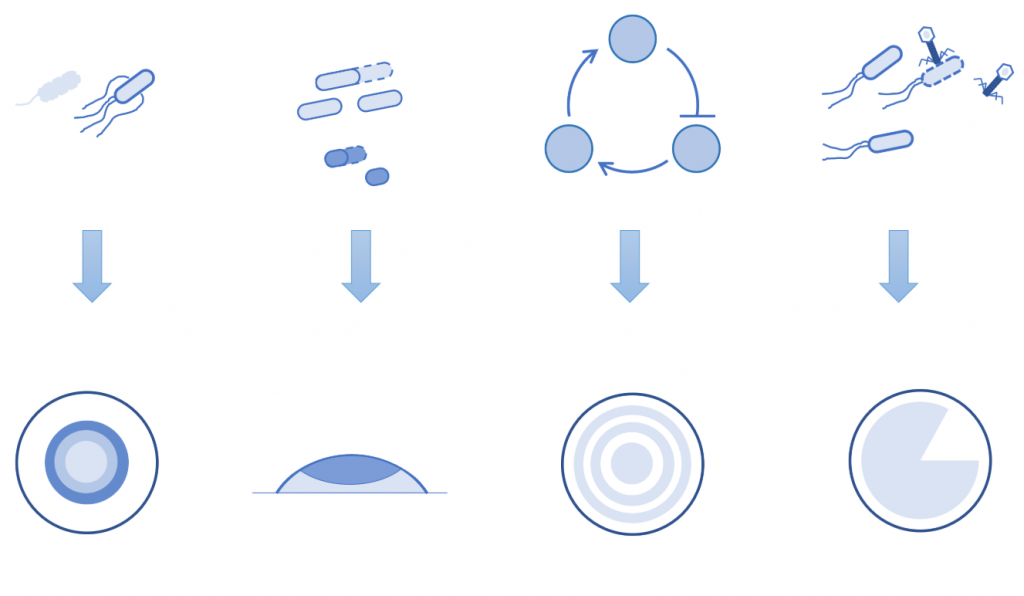
Understanding the fundamental principles governing spontaneous biological pattern formation is a central challenge in developmental biology, nonlinear physics, and synthetic biology. These principles not only deepen our knowledge of embryogenesis and tissue morphogenesis but also hold transformative potential for regenerative medicine and programmable tissue engineering. Traditional theories of pattern formation often rely on static boundary conditions to explain how cell differentiation generates non-uniform structures during early development. However, biological systems are inherently dynamic—cells proliferate, tissues expand, and spatial boundaries evolve, leading to complex symmetry breaking and the emergence of novel patterns.
To decipher these dynamic processes, our lab employs microbial population expansion as a model system, combining synthetic biology tools and a “build-to-understand” approach. By rationally engineering synthetic gene circuits and observing their behavior in spatially expanding populations, we aim to uncover universal design principles that govern biological pattern formation. Our work bridges theory and experiment, revealing how simple genetic interactions can give rise to intricate, self-organized structures.
Key Insights from Our Research:
- Spatial Self-Organization: We demonstrated how sequential stripe patterns emerge in expanding cell populations (Liu et al., Science 2011), providing a blueprint for synthetic pattern design.
- Conflict Resolution in Collective Migration: By studying bacterial groups, we revealed how spatial organization balances individuality and collective movement (Fu et al., Nature Communications 2018).
- Ordered Phenotypic Diversity: We showed how modulated individual behaviors can produce structured group migration patterns (Bai et al., eLife 2021).
- Navigated Range Expansion: Recent work uncovered how environmental constraints shape migratory culling and pattern robustness (Zhang et al., PNAS 2025).
- Multistability in Colony Patterns: Engineered bistable switches were shown to generate diverse, coexisting colony architectures (Chu et al., PNAS 2025).
Our goal is to synthesize these insights into a “Biological Space Expansion Pattern Diagram”—a predictive framework that maps genetic circuit design to spatial outcomes under dynamic growth conditions. This framework will empower the engineering of programmable tissues, biofilms, and synthetic ecosystems with precision. By integrating computational modeling, microfabrication, and real-time imaging, we aim to transform pattern formation from an observational science into a design discipline with applications in biomedicine, agriculture, and beyond.
Compatibility Rules Between Synthetic Gene Circuits and Chassis Cells
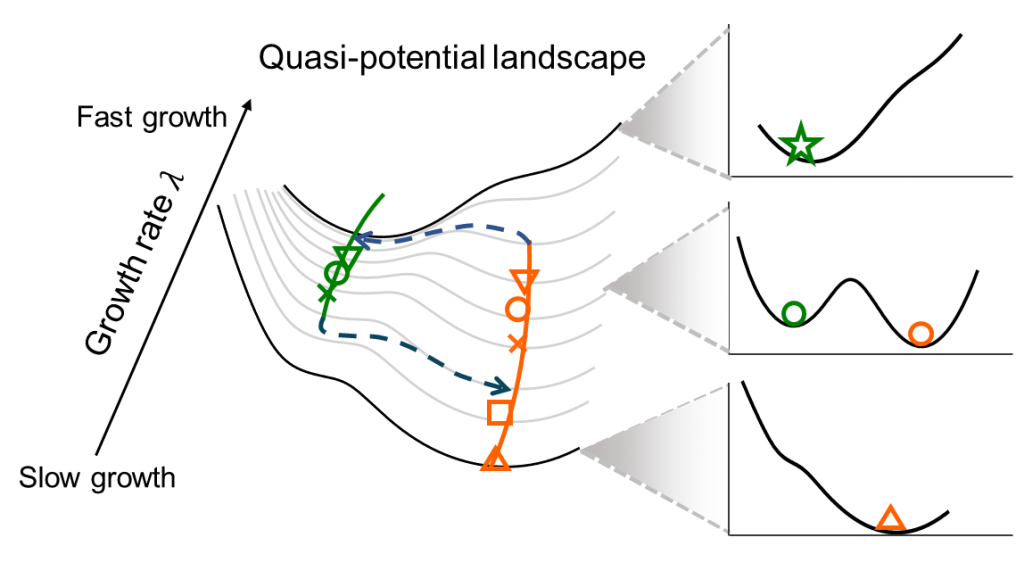
Cellular differentiation and organismal development are orchestrated by intricate gene regulatory networks (GRNs) that respond to intrinsic topological structures and physiological constraints. These endogenous networks operate within the dynamic context of cellular physiology, where protein allocation and metabolic flux distribution influence global growth rates and, consequently, phenotypic outcomes. Understanding how synthetic gene circuits interact with host cellular machinery is essential for reliable bioengineering, as mismatches between circuit design and chassis physiology can lead to unpredictable behavior.
Our research investigates the compatibility rules governing synthetic gene circuits and their bacterial chassis, using Escherichia coli as a model system. By constructing bistable toggle switches, we have demonstrated how growth rate variations can drive transitions between monostable and bistable states, reshaping cellular decision-making landscapes. These findings reveal that synthetic circuits are not isolated modules but are deeply intertwined with host physiology, where metabolic and proteomic constraints dictate circuit performance. Our work highlights the need for context-aware circuit design, ensuring robust functionality across diverse cellular conditions.
Key publications from our lab include:
- Zhu J.#, Chu P.#, Fu X. (2023)* Unbalanced response to growth variations reshapes the cell fate decision landscape. Nature Chemical Biology 19, 1097–1104. DOI:10.1038/s41589-023-01346-x
- Chu P.#, Zhu J.#, Ma Z, Fu X. (2025)* Colony pattern multistability emerges from a bistable switch. Proceedings of the National Academy of Sciences 122(14), e2424112122. DOI:10.1073/pnas.2424112122
By elucidating the interplay between synthetic circuits and host physiology, we aim to establish design principles for predictable and scalable genetic engineering, advancing applications in biotechnology, therapeutics, and synthetic biology.